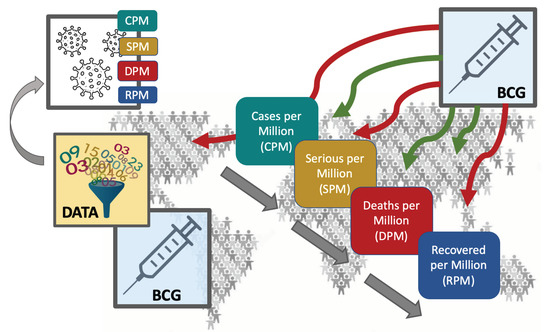
Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vaccination Coverage: A Multivariable Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Data Analysis and Statistical Tests
3. Results
3.1. BCG Administration Years are Negatively Correlated with COVID-19 Outcomes
3.2. Multivariable Analysis Reveals a Strong Contribution of BCG Administration to the COVID-19 Outcome Statistics
3.3. Highest Correlation with BCG Age Coverage Applies to the Most Recently Vaccinated
3.4. Data and Materials Availability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Reported Estimates of BCG Coverage; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Dowd, J.B.; Andriano, L.; Brazel, D.M.; Rotondi, V.; Block, P.; Ding, X.; Liu, Y.; Mills, M.C. Demographic science aids in understanding the spread and fatality rates of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 9696–9698. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.H.; Ahmed, Q.A.; Gozzer, E.; Schlagenhauf, P.; Memish, Z.A. Covid-19 and community mitigation strategies in a pandemic. BMJ 2020, 368, m1066. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-m.; Akhmetzhanov, A.R.; Hayashi, K.; Linton, N.M.; Yang, Y.; Yuan, B.; Kobayashi, T.; Kinoshita, R.; Nishiura, H. Real-time estimation of the risk of death from novel coronavirus (COVID-19) infection: Inference using exported cases. J. Clin. Med. 2020, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020, 92, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020, 395, 931–934. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldog, P.; Tekeli, T.; Vizi, Z.; Denes, A.; Bartha, F.A.; Rost, G. Risk Assessment of Novel Coronavirus COVID-19 Outbreaks Outside China. J. Clin. Med. 2020, 9, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbaraman, N. Why daily death tolls have become unusually important in understanding the coronavirus pandemic. Nature 2020. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Gilbert, M.; Pullano, G.; Pinotti, F.; Valdano, E.; Poletto, C.; Boelle, P.Y.; D’Ortenzio, E.; Yazdanpanah, Y.; Eholie, S.P.; Altmann, M.; et al. Preparedness and vulnerability of African countries against importations of COVID-19: A modelling study. Lancet 2020, 395, 871–877. [Google Scholar] [CrossRef] [Green Version]
- Orme, I.M. Beyond BCG: The potential for a more effective TB vaccine. Mol. Med. Today 1999, 5, 487–492. [Google Scholar] [CrossRef]
- Brewer, T.F.; Colditz, G.A. Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin. Infect. Dis. 1995, 20, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Glaziou, P.; Sismanidis, C.; Floyd, K.; Raviglione, M. Global epidemiology of tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareek, M.; Greenaway, C.; Noori, T.; Munoz, J.; Zenner, D. The impact of migration on tuberculosis epidemiology and control in high-income countries: A review. BMC Med. 2016, 14, 48. [Google Scholar] [CrossRef] [Green Version]
- Moorlag, S.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- WorldMeters. Published Online at Worldmeters.info, Dover. Available online: https://www.worldometers.info/coronavirus/ (accessed on 21 May 2020).
- van Rossum, G. Python Tutorial, Technical Report CS-R9526, 1st ed.; Centrum Voor Wiskunde En Informatica (CWI): Amsterdam, The Netherlands, 1995. [Google Scholar]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG World Atlas: A database of global BCG vaccination policies and practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef] [Green Version]
- Bank, W. Population Ages 65 and Above (% of Total Population). Available online: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS/ (accessed on 18 April 2020).
- Basilaia, G.; Kvavadze, D. Transition to online education in schools during a SARS-CoV-2 coronavirus (COVID-19) pandemic in Georgia. Pedagog. Res. 2020, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Kelvin, A.A.; Halperin, S. COVID-19 in children: The link in the transmission chain. Lancet Infect. Dis. 2020, 20, 633–634. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zhang, J.; Zeng, M.; Yun, Q.; Guo, W.; Zheng, Y.; Zhao, S.; Wang, M.H.; Yang, Z. Epidemiological parameters of coronavirus disease 2019: A pooled analysis of publicly reported individual data of 1155 cases from seven countries. medRxiv 2020. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.; Kupferschmidt, K. Countries test tactics in ‘war’ against COVID-19. Am. Assoc. Adv. Sci. 2020, 367, 1287–1288. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Hopman, J.; Allegranzi, B.; Mehtar, S. Managing COVID-19 in low-and middle-income countries. JAMA 2020, 323, 1549–1550. [Google Scholar] [CrossRef]
- Viner, R.M.; Russell, S.J.; Croker, H.; Packer, J.; Ward, J.; Stansfield, C.; Mytton, O.; Bonell, C.; Booy, R. School closure and management practices during coronavirus outbreaks including COVID-19: A rapid systematic review. Lancet Child Adolesc. Health 2020, 4, 397–404. [Google Scholar] [CrossRef]
- Pan, A.; Liu, L.; Wang, C.; Guo, H.; Hao, X.; Wang, Q.; Huang, J.; He, N.; Yu, H.; Lin, X. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA 2020, 323, 1915. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; group, C.C.-W.; Eggo, R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, J.; Hong, L.; Luo, Y.; Song, Q.; Chen, D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: An observational cohort study. Lancet Infect. Dis. 2020, 20, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gu, Z.; Xia, S.; Shi, B.; Zhou, X.N.; Shi, Y.; Liu, J. What are the underlying transmission patterns of COVID-19 outbreak? –An Age-specific Social Contact Characterization. EClinicalMedicine 2020, 22, 100354. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.; Tariq, A.; Choi, W.; Lee, Y.; Chowell, G. Transmission potential and severity of COVID-19 in South Korea. Int. J. Infect. Dis. 2020, 93, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gursel, M.; Gursel, I. Is Global BCG vaccination coverage relevant to the progression of SARS-CoV-2 pandemic? Med. Hypotheses 2020, 109707. [Google Scholar] [CrossRef]
- Bloom, B.R. BCG: Its Impact on Tuberculosis and Relevance to Autoimmune Disease. In The Value of BCG and TNF in Autoimmunity; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–10. [Google Scholar]
- Butkeviciute, E.; Jones, C.E.; Smith, S.G. Heterologous effects of infant BCG vaccination: Potential mechanisms of immunity. Future Microbiol. 2018, 13, 1193–1208. [Google Scholar] [CrossRef] [Green Version]
- Linehan, M.F.; Frank, T.L.; Hazell, M.L.; Francis, H.C.; Morris, J.A.; Baxter, D.N.; Niven, R.M. Is the prevalence of wheeze in children altered by neonatal BCG vaccination? J. Allergy Clin. Immunol. 2007, 119, 1079–1085. [Google Scholar] [CrossRef]
- Salman, S.; Ahmed, M.S.; Ibrahim, A.M.; Mattar, O.M.; El-Shirbiny, H.; Sarsik, S.; Afifi, A.M.; Anis, R.M.; Agha, N.A.Y.; Abushouk, A.I. Intralesional immunotherapy for the treatment of warts: A network meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 922–930. [Google Scholar] [CrossRef]
- Bluhm, R.; Pinkovskiy, M. The spread of COVID-19 and the BCG vaccine: A natural experiment in reunified Germany. FRB N. Y. Staff Rep. 2020, 5, 926. [Google Scholar] [CrossRef]
- Bodova, K.; Boza, V.; Brejova, B.; Kollar, R.; Mikusova, K.; Vinar, T. Time-adjusted analysis shows weak associations between BCG vaccination policy and COVID-19 disease progression. MedRxiv 2020. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Netea, M.G. BCG-induced trained immunity: Can it offer protection against COVID-19? Nat. Rev. Immunol. 2020, 20, 335–337. [Google Scholar] [CrossRef]
- Netea, M.G.; van Crevel, R. BCG-induced protection: Effects on innate immune memory. Semin. Immunol. 2014, 26, 512–517. [Google Scholar] [CrossRef]
- Dockrell, H.M.; Smith, S.G. What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018, 172, 176–190. [Google Scholar] [CrossRef] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, P.D.; Sutherland, I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br. Med. J. 1977, 2, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Salman, S.; Salem, M.L. Routine childhood immunization may protect against COVID-19. Med. Hypotheses 2020, 140, 109689. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, R.; Villarreal-Ramos, B.; Vordermeier, H.M.; McShane, H. The humoral immune response to bcg vaccination. Front. Immunol. 2019, 10, 1317. [Google Scholar] [CrossRef] [Green Version]
- Angelidou, A.; Diray-Arce, J.; Conti, M.G.; Smolen, K.K.; Van Haren, S.D.; Dowling, D.J.; Husson, R.N.; Levy, O. BCG as a case study for precision vaccine development: lessons from vaccine heterogeneity, trained immunity, and immune ontogeny. Front. Microbiol. 2020, 11, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkman, K.; Sombroek, C.C.; Vervenne, R.A.W.; Hofman, S.O.; Boot, C.; Remarque, E.J.; Kocken, C.H.M.; Ottenhoff, T.H.M.; Kondova, I.; Khayum, M.A.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef]
- Wardhana, D.E.; Sultana, A.; Mandang, V.V.; Jim, E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta. Med. Indones 2011, 43, 185–190. [Google Scholar] [PubMed]
- de Vriese, J. Can a century-old TB vaccine steel the immune system against the new coronavirus. Science 2020. [Google Scholar] [CrossRef]
- Ayoub, B.M. COVID-19 vaccination clinical trials should consider multiple doses of BCG. Die Pharm. 2020, 75, 159. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinger, D.; Blass, I.; Rappoport, N.; Linial, M. Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vaccination Coverage: A Multivariable Analysis. Vaccines 2020, 8, 378. https://doi.org/10.3390/vaccines8030378
Klinger D, Blass I, Rappoport N, Linial M. Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vaccination Coverage: A Multivariable Analysis. Vaccines. 2020; 8(3):378. https://doi.org/10.3390/vaccines8030378
Chicago/Turabian StyleKlinger, Danielle, Ido Blass, Nadav Rappoport, and Michal Linial. 2020. "Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vaccination Coverage: A Multivariable Analysis" Vaccines 8, no. 3: 378. https://doi.org/10.3390/vaccines8030378